Welcome to Dongguan Yinso Medical Packaging Co.,Ltd.
Welcome to Dongguan Yinso Medical Packaging Co.,Ltd.

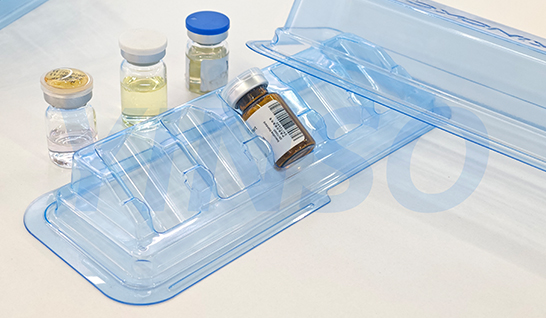
Manufacturers must ensure that medical device packaging and shipping containers protect devices from deformation or damage under typical processing, storage, handling, and distribution conditions. Design considerations also impact material selection suitable for the device. Often, packaging merely holds the product. However, for medical devices, packaging has specific applications. Comprehending the product's purpose, dimensions, shape, unique characteristics (e.g., sharp edges, points, fragility), and distribution environment, especially antibacterial requirements, is crucial for selecting suitable materials and successfully designing the final packaging.
Here are six steps Yinso Medical Packing suggests for developing medical device packaging:
Step 1: Consult Regulations
Medical device manufacturers should consult their regulatory affairs department to ensure packaging complies with FDA or other international regulations.
Step 2: Compile Packaging Design Requirements
Packaging engineers must collaborate with project team members to create a packaging design requirements document.
Step 3: Establish a Compliant Packaging System
Protective packaging ensures devices remain sterile until use. The packaging system must prevent any breaches in the sterile barrier (for sterile barrier packaging) and prevent damage to medical devices, which can be unpredictable and affect device safety and effectiveness.
Step 4: Review Labeling Requirements
General labeling rules outline minimum requirements for all devices. 21 CFR Part 80 regulates medical device labeling, including current Sections A, C, D, E, and H. Familiarize with mandatory requirements in Section A (General Labeling Provisions) and check other sections for specific requirements.
Step 5: Conduct Distribution and Use Testing
With increasingly complex medical devices, packaging designers must understand anticipated distribution, storage, and use conditions. Testing typically follows published standards in simulated distribution environments.
Step 6: Perform Stability Testing
Accelerated and real-time aging tests for sterile barrier systems. For medical devices, the industry follows ISO 11607 (1) and (2):2006, which mandates stability testing for sterile barrier systems. Manufacturers worldwide conduct shelf-life studies to support product and packaging (sterile) effectiveness. Through accelerated and real-time aging protocols, test the overall packaging and seal integrity at specific intervals to assess aging-related phenomena and seal integrity.
info@yinsopack.com
+86 15014837000(Wechat/WhatsApp/Skype)
NO.59 Meilin Road, Dalingshan Town, Dongguan City,Guangdong Province, China
Get a Free Prototype + Compliance Report Within 5 Days

Yinso Packing has been a leading figure in the medical packaging industry since 2007. We are committed to providing reliable, cost - effective, and innovative rigid thermoformed packaging solutions to top medical companies.
© 2025 Dongguan Yinso Medical Packaging Co.,Ltd All Rights Reserved.
AddressNO.59 Meilin Road, Dalingshan Town, Dongguan City,Guangdong Province, China
Emailinfo@yinsopack.com
Tel(Wechat/WhatsApp/Skype)+86 15014837000
About Us Medical Blister Packs Products Capabilities Blogs Contact Us